
Lithium-ion batteries are a type of rechargeable battery that has been widely used in consumer electronics and electric vehicles in recent decades. They are known for their high energy density, low self-discharge, and relatively low maintenance compared to other types of batteries.
The history of lithium-ion batteries dates back to the early 1970s, when a team of scientists at Oxford University first observed the potential for lithium ions to be used as a charge-carrying medium in batteries. However, it wasn’t until the 1980s that the first practical lithium-ion batteries were developed.
In 1980, Dr. John Goodenough, a professor at the University of Texas at Austin, discovered that cobalt oxide could be used as the cathode in a lithium-ion battery, which significantly increased the battery’s energy density. A few years later, in 1985, Dr. Rachid Yazami, a scientist at the National University of Singapore, developed a graphite anode for lithium-ion batteries, which improved their stability and safety.






Since then Lithium-ion batteries have become an essential component in modern technology and transportation.
The first research on rechargeable Li-ion batteries dates back to the 1960s, with one of the earliest examples being a CuF2/Li (Copper monofluoride) battery developed by NASA in 1965.
In the 1970s, British chemist M. Stanley Whittingham made a significant breakthrough by using titanium disulfide (TiS2) as a cathode material in a lithium-ion battery.
Oil giant Exxon tried commercialise the technology but were unsuccessful due to the high cost and complexity of synthesizing TiS2, as well as the batteries’ tendency to catch fire due to the presence of metallic lithium.
A more significant breakthrough came in 1980 when, Ned A. Godshall, Koichi Mizushima, and John B. Goodenough each independently developed lithium-ion batteries using lithium cobalt oxide (LiCoO2) as the cathode material, which offered a higher voltage and was more stable in air.
Moroccan inventor Rachid Yazami, working out of Singapore also made an important contribution that same year by demonstrating the reversible electrochemical intercalation of lithium in graphite and inventing the lithium graphite electrode (anode).
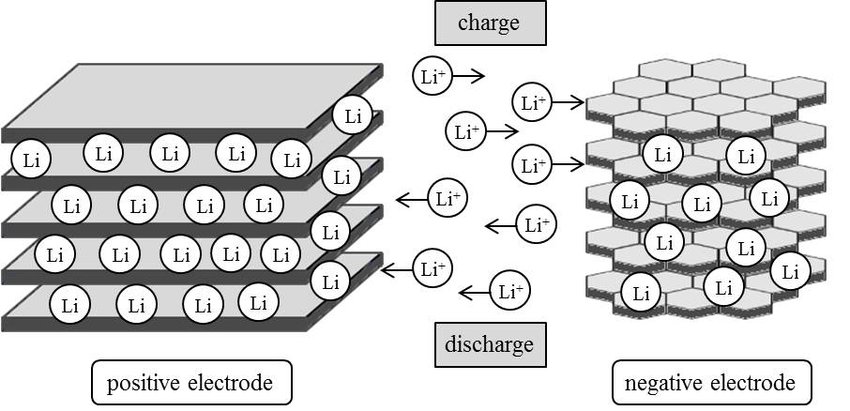
Intercalation is a process in which a substance is inserted or “intercalated” into the spaces or “interstices” between the layers of another substance. It is often used in the context of batteries, where intercalation refers to the insertion of ions (charged atoms or molecules) into the structure of a cathode or anode material.
The early batteries with metallic lithium were abandoned due to safety concerns and complexity in manufacturing.
In 1987, Akira Yoshino patented the first commercial lithium-ion battery using a “soft carbon” anode, Goodenough’s LiCoO2 cathode, and a carbonate ester-based electrolyte.
Sony began producing and selling this type of battery in 1991, and the following year, a joint venture between Toshiba and Asashi Kasei Co. also released their own version. Most of these early batteries were used in high powered mobile devices such as digital cameras and video cameras that require stable voltage to operate.

In the 1990s, energy density was improved by replacing the soft carbon anode with hard carbon and later with graphite.
In 2012, John B. Goodenough, Rachid Yazami, and Akira Yoshino received the IEEE Medal for Environmental and Safety Technologies for their work on lithium-ion batteries.
Goodenough, Whittingham, and Yoshino were also awarded the Nobel Prize in Chemistry in 2019 for the development of lithium-ion batteries.

Since then, lithium-ion batteries have undergone numerous technological advancements and improvements, and they have become the dominant type of rechargeable battery in many applications. Today, key players in the lithium-ion battery industry include companies such as Tesla, Panasonic, LG Chem, and Samsung.
In the late 1990s and early 2000s, lithium-ion batteries became widely used in portable electronic devices such as laptops, cell phones, and digital cameras.
In the 2010s, lithium-ion batteries began to be used in a wider range of applications, including electric vehicles (EVs). The increasing demand for EVs, which require large, high-capacity batteries, has further spurred the development of lithium-ion battery technology.

In recent years, researchers and companies have been working on improving the performance, safety, and sustainability of lithium-ion batteries. This has included efforts to increase the energy density of the batteries, reduce their cost, and develop more sustainable materials and manufacturing processes.
A particularly popular version of the lithium ion recipe is the lithium iron phosphate battery that is very popular among China automakers.
Lithium iron phosphate (LiFePO4) batteries are a type of lithium-ion battery that uses lithium iron phosphate as the cathode material. They are also known as LFP batteries or LiFePO4 batteries.

Ternary batteries, on the other hand, are lithium-ion batteries that use a cathode material made of a mixture of cobalt, nickel, and manganese, often referred to as a “ternary” material.
Here are some key differences between LiFePO4 batteries and ternary batteries in terms of chemistry, performance, cost, and safety:
Chemistry: The main difference between these two types of batteries is the cathode material used. LiFePO4 batteries use lithium iron phosphate as the cathode, while ternary batteries use a mixture of cobalt, nickel, and manganese. The cathode material can have a significant impact on the performance and properties of the battery.
Performance: LiFePO4 batteries tend to have a lower energy density and a higher voltage than ternary batteries. This means that they can store less energy per unit of weight, but they can deliver it at a higher voltage. Ternary batteries, on the other hand, have a higher energy density and a lower voltage, which makes them more suitable for applications that require a lot of energy storage, such as electric vehicles.
Cost: LiFePO4 batteries are generally less expensive to produce than ternary batteries because they use less expensive materials. However, the cost of lithium-ion batteries can vary depending on a variety of factors, such as the raw material prices, production costs
Safety: LiFePO4 batteries are generally considered to be more stable and safer than ternary batteries. This is because lithium iron phosphate has a higher thermal stability and a lower risk of thermal runaway, which is a phenomenon that can occur when a battery overheats and self-ignites. Ternary batteries, on the other hand, have a higher risk of thermal runaway due to the presence of cobalt in the cathode material.

Additionally, LiFePO4 batteries have a slower rate of degradation and a longer lifespan than ternary batteries, which makes them more suitable for applications where the battery will be used over an extended period of time.
That said, all lithium-ion batteries, including both LiFePO4 and ternary batteries, require proper handling and use to ensure their safe operation. This includes following recommended charging and discharging protocols, avoiding physical damage to the battery, and using safety features such as overcharge protection and short circuit protection.
China has been a major player in the development and production of lithium iron phosphate (LiFePO4) batteries for a number of years.
John B. Goodenough, is credited with the discovery of lithium iron phosphate (LiFePO4) as a cathode material for lithium-ion batteries.
In the late 1990s and early 2000s, Goodenough and his team at the University of Texas at Austin published several papers on the use of LiFePO4 as a cathode material in lithium-ion batteries, which demonstrated its potential for high-performance, low-cost energy storage.
It is difficult to pinpoint an exact timeline, but it is likely that China began actively researching and developing LiFePO4 battery technology about the same time Goodenough made the discovery in the early to mid-2000s.
In recent years, China has significantly expanded its production capacity for LiFePO4 batteries, particularly for use in electric vehicles (EVs). This expansion has been driven by the growing demand for EVs in the country, as well as the supportive policies and incentives provided by the Chinese government.
Today, China is a global leader in the production and export of LiFePO4 batteries, with a significant share of the global market. However, it is worth noting that LiFePO4 battery technology is also being developed and used in other parts of the world, including in Europe, North America, and Asia.
Today, global lithium-ion battery production capacity is at 767 GWh, with China accounting for 75%. Production is estimated to be between 200 and 600 GWh in 2021, and predictions for 2023 range from 400 to 1,100 GWh.
Global battery demand is expected to be as high as 10TWh in 2030 if electric cars and renewable energy continues to follow the current uptake trends.

